The NIHR are excited to announce the stages of the NIHR GCP programme launch, designed to strengthen research delivery and ensure compliance with the updated ICH GCP E6(R3) guidelines.
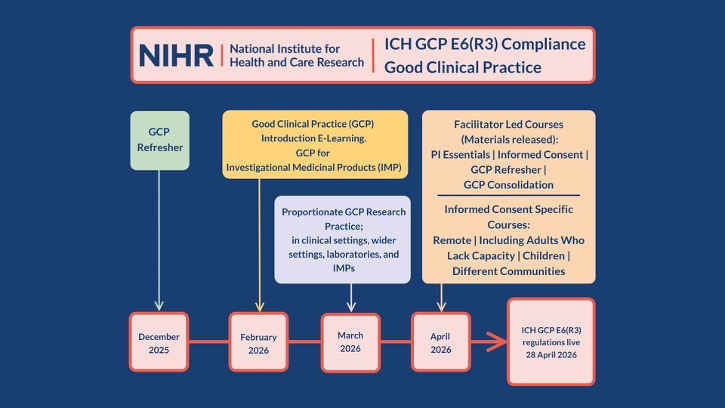
December 2025: NIHR’s first GCP E6(R3) learning is available
The GCP E6(R3) Refresher will launch on Monday 8th December.
A key opportunity for experienced research delivery staff to refresh their knowledge and lead the way in implementing the changes introduced in the update to the UK Clinical Trials Regulations and GCP E6(R3). Understanding these updates firsthand will enable you to help set the standard for quality and compliance across our research community.
Access to this course will be found on the NIHR GCP Page when it is launched.
February 2026: Building Strong Foundations
February sees the launch of:
- Good Clinical Practice (GCP) Introduction E-Learning – This course is for staff new to Research Delivery.
- GCP for Investigational Medicinal Products (IMP) – An additional training offer for staff who are working on CTIMPs and managing IMPs.
March 2026: Proportionate Approaches to Research Training
March takes a deeper dive into the NIHR proportionate training offer through the ‘Research Practice’ courses. These modules are tailored for non-CTIMPs and observational research, and extend to broader contexts and settings. They also support those working in laboratories and managing IMPs while supporting research. Importantly, the courses consider staff who assist research within their normal role without being delegated research-specific activities, ensuring training is relevant and practical.
- Research Practice in Clinical Settings
- Research Practice in Wider Health, Care and Community Settings
- Research Practice in IMP Management
- Research Practice in Laboratories
These courses will help ensure that research is conducted efficiently and appropriately, no matter the research trial type or setting.
April 2026: Informed Consent Specialist Modules and Updated Facilitator-Led Courses
April is packed with exciting updates to the following Informed Consent E-Learning courses:
- Remote Consent
- Consent for Adults Who Lack Capacity
- Consent with Children
- Informed Consent with Different Communities
April will also see the release of updated facilitator materials to deliver the following courses;
- GCP Consolidation
- GCP Refresher
- Informed Consent
- PI Essentials
Research Ready: The timeline of NIHR GCP E6(R3) Training Launches
Big changes are on the horizon. From refreshed GCP modules to specialist courses, the NIHR programme is designed to keep you research-ready and compliant with the latest standards. Stay tuned and explore the upcoming launches.
